Advances in neuroscience are closely linked to advances in cell imaging. The invention of the microscope in the 17th century first allowed the characterization of the human brain at the cellular level, and clinical neurology, neurosurgery and psychiatry emerged in the 18th and 19th centuries. Similarly, the understanding and classification of neurodegenerative diseases benefited from the development of immunostaining techniques and multichannel fluorescence imaging in the second half of the 20th century. However, despite these advances, diffraction barriers remain a resolution limitation of traditional light microscopy, hindering the accurate characterization of subcellular structures. In conventional fluorescence microscopy, all illuminated molecules emit signals simultaneously within the diffraction-limited volume, limiting the spatial resolution of the final acquired image. ”
In recent years, super-resolution microscopy has overcome this obstacle, using different methods to distinguish fluorescent molecules located within the same diffraction-limited volume. One of them, called stochastic optical reconstruction microscopy (STORM), is based on single-molecule localization by randomly turning on light-switched fluorescent molecules within a diffraction-limited volume at different time points so their signals largely do not overlap. When isolated in space, the position of each individual fluorophore can be determined by locating the center of its signal. Super-resolution images are then generated from numerous molecular localization accumulated over time, providing a spatial resolution of < 50 nm.
In the field of neuroscience, this new technique has led to the characterization of the periodic structure of neuronal axon cytoskeleton and the spatial organization of synaptic proteins. In this study, researchers will use cell imaging techniques combined with neuropathological techniques to perform 2D, 3D, and bicolor STORM on human brain samples from control subjects and patients with common age-related neurodegenerative diseases.
《STochastic Optical Reconstruction Microscopy (STORM) reveals the nanoscale organization of pathological aggregates in human brain》
NO.1 Research results (excerpt)
1. Use STORM to super-resolution imaging of human brain samples
To evaluate STORM imaging of human brain tissue, we first aimed to resolve its histological structure, such as the neocortical axon tract. Frozen samples from the prefrontal cortex of control subjects were cut by standard cryostat methods and immunostained with antifilament primary antibodies and secondary antibodies conjugated to Alexa Fluor (AF) 647. Place coverslips with immunostained brain sections on a stage equipped with a high-power laser module, a total internal reflection fluorescence (TIRF) system, and an electron multiplication charge-coupled device (EMCCD) single-photon-sensitive inverted motorized microscope (Figure 1A).
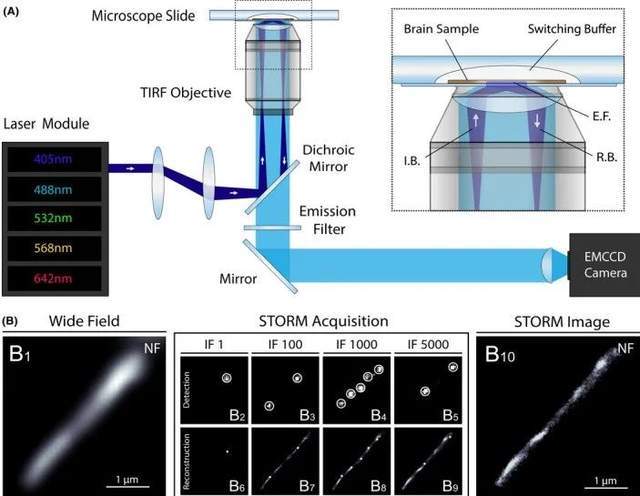 Figure 1. Super-resolution imaging of human brain samples using STORM
Figure 1. Super-resolution imaging of human brain samples using STORM
STORM acquires cortical axons of neurofilament (NF) immunostaining in human brain slices: traditional wide-field fluorescence microscopy images (B1) are first obtained, followed by a strong increase in excitation power to induce fluorophore scintillation and produce thousands of frames (B2-B5). Detect the localization of activated fluorescent molecules with subpixel precision (B6-B9) on a per-frame basis. The super-resolution image (B10) is then reconstructed using cumulative positioning from all frames.
2. 3D-STORM and two-color STORM imaging of human brain samples
Three-dimensional (3D) and multichannel imaging make it possible to provide insight into the structural organization of nanoscale structures. To perform 3D-STORM in slices of the human brain, the researchers used an astigmatism-based method. After setting the cylindrical lens in the detection path of the microscope, the z-coordinate of each fluorescent molecule is determined from the ovality of its image. Neurofilaments (NF) were immunostained with prefrontal cortical samples from control subjects, and after reconstruction, axons were visualized in 3D with high resolution and imaging depth of approximately 0.5 μm (Figure 2A). The cylindrical shape of the axon bundle is recognizable, and the dimensions of the longitudinal and transverse sections of the 3D modeled axon are similar to those obtained by 2D-STORM.
Then, to evaluate bicolor STORM imaging of cortical samples, the investigators immunostained human brain sections with two primary antibodies that detect nearby structures (presynaptic and postsynaptic bassoon tubes and Homer 1 protein) and secondary antibodies conjugated to the photoswitch fluorophores AF 647 and AF 532 (Figure 2B). Under conventional fluorescence microscopy, the bassoon tube and Homer 1 signals diffuse and overlap, and synaptic gaps cannot be precisely defined. In contrast, bicolor STORM accurately distinguishes presynaptic and postsynaptic protein clusters separated by synaptic clefts and defines the size, orientation, and organization of synapses. Interestingly, multichannel imaging combining traditional widefield fluorescence microscopy and STORM can also be achieved, allowing super-resolution imaging of brain structures using light-switchable and non-light-switchable fluorophores. Although not as specific as bicolor STORM, this technique provides valuable information about the layout of adjacent structures, such as the myelin sheath around the axon bundle (Figure 2C). The size of the super-resolution structure is comparable to that measured using TEM in the same area (Figure 2B3, C3).
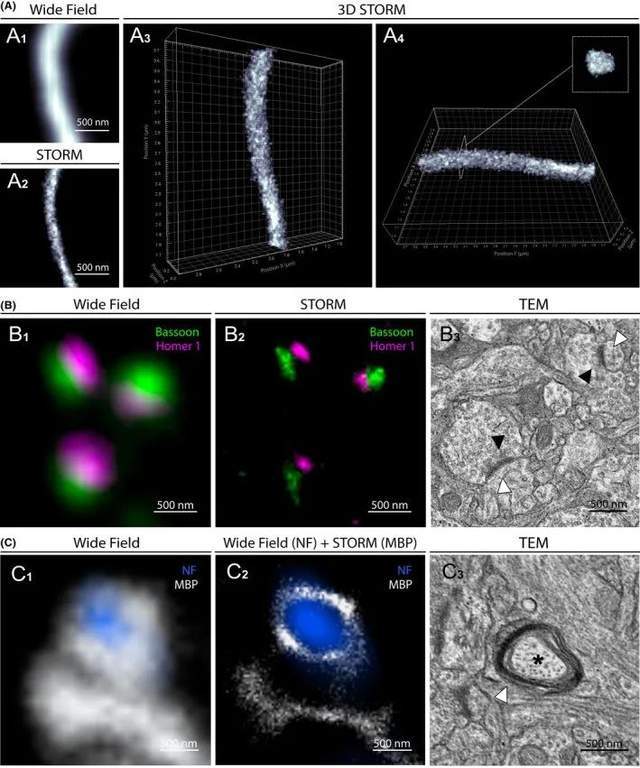 Figure 2.3D-STORM and bicolor STORM images of the physiological structure of the human brain
Figure 2.3D-STORM and bicolor STORM images of the physiological structure of the human brain
3. STORM imaging of amyloid-β and TAU protein pathies
Alzheimer's disease (AD) is the leading cause of dementia. The two main hallmarks of the disease are extracellular deposits of Aβ peptides, some of which form the core of senile plaques, and intraneuronal aggregates of hyperphosphorylated tau protein (p.Tau) called neurofibrillary tangles (NFTs). Since aggregates can be up to 100 μm in size, maintaining a holistic view of the entire lesion is essential to study Aβ and Tau pathology in the brain, while high-resolution imaging is essential for characterizing nanoscale tissues of misfolded proteins.
The researchers used STORM to image the entire age plaque and degenerative neurons. Tissue samples from the prefrontal, parietal, and temporal cortex of AD patients were immunostained for Aβ and p.Tau (Ser202 phosphate, Thr205). STORM images of age spots with ~30 μm diameter and ~15 μm of degenerate neurons with NFTs were obtained. While paired spiral filaments of Aβ fibrils and Tau cannot be identified like TEM, the STORM image provides high-resolution details of the nanoscale distribution and size of Aβ and p. Tau aggregates (Figure 3). Intraneuronal p.Tau NFTs appear denser, with honeycomb-like structures in the soma and filamentous tissue in axons. The presence of unstained spots in aggregates indicates the inclusion of other components such as proteins or organelles. These results highlight the usefulness of STORM for imaging Aβ and p.Tau aggregates in brain samples from AD patients at high resolution.
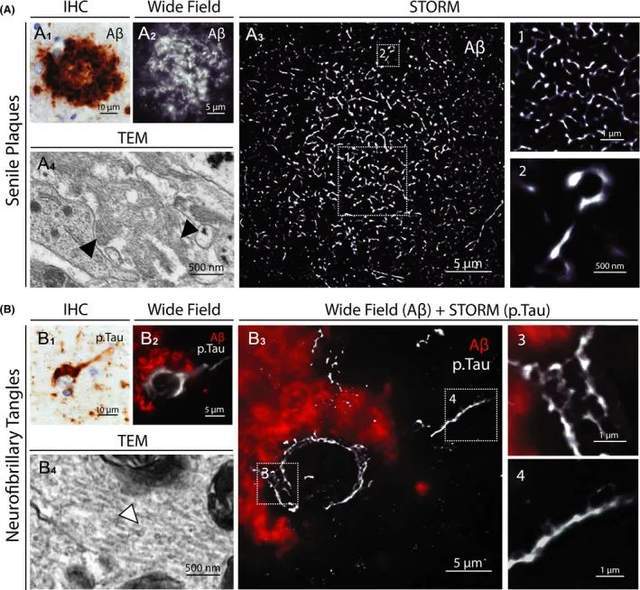 Figure 3.3D-STORM and bicolor STORM images of the physiological structure of the human brain
Figure 3.3D-STORM and bicolor STORM images of the physiological structure of the human brain
4. STORM imaging of Louis pathology
Parkinson's disease (PD) and dementia with Lewy bodies (DLB) are two neurodegenerative diseases characterized by the presence of intraneuronal phosphorylated α-synuclein (p.α-syn) immunoreactive inclusion bodies called Lewy bodies (LB). The structure of LBs varies according to their localization in the central nervous system. Two main types of LB can be observed through immunohistochemistry: the typical Lewy body (TLB), which has a pale core surrounded by dense halos mainly in the brainstem, and the smaller cortical Lewy body (CLB), which lacks a central core and is mainly detected in the neocortex. The accumulation of p.α-syn in a dystrophic axon called the Lewy axon (LN) can also be observed. To date, the structure of LB remains unclear because the resolution of conventional fluorescence microscopy is too low to characterize its internal structure, and TEM does not provide sufficient information about its protein content and organization.
To characterize LB tissue at the nanoscale using molecular staining, the investigators performed STORM imaging on brain samples from PD and DLB patients. Immunostaining of substantia nigra and prefrontal cortex sections with anti-p.α-syn (phosphorylated Ser129) antibodies and images of TLB, CLB, and LN were obtained. Although only STORM images precisely define their structure, the ring-shaped appearance of TLBs is observed in both routine and super-resolution imaging (Figure 4A). The pale core appears unstained, while the dense halo at the periphery is made of reticular p.α-syn. Similarly, STORM imaging of CLB reveals dense cellular structures that cannot be observed with conventional fluorescence microscopy (Figure 4B, 4C). As for p. Tau aggregates, the unstained cores and spots observed in LB may correspond to protein partners or trapped organelles.
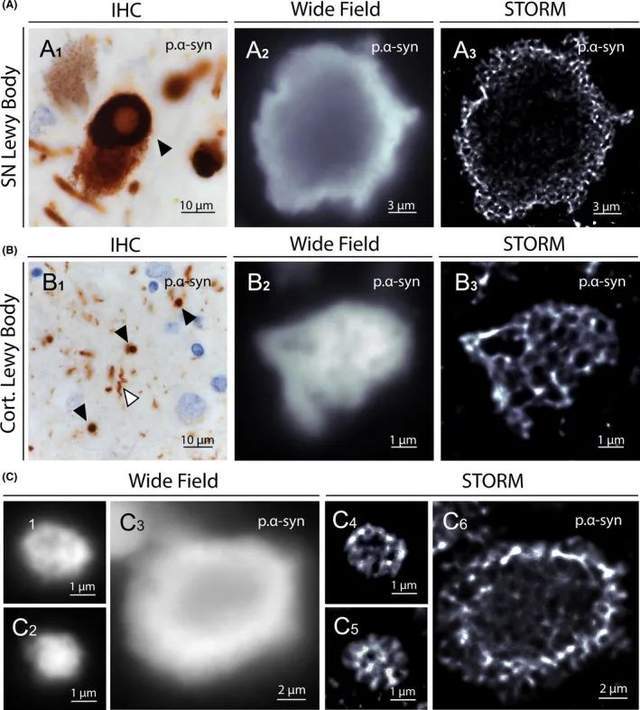 Figure 4. STORM images of Lewy bodies in brain samples from PD and DLB patients
Figure 4. STORM images of Lewy bodies in brain samples from PD and DLB patients
In fact, Lewy bodies are multiprotein complexes made up of more than 100 proteins, including p.tau. Therefore, we performed bicolor STORM imaging of LB using antibodies against p.Tau and p.α-syn to precisely define the internal structure of the lesion and clearly distinguish one protein from another (Figure 5A). STORM imaging accurately measured the width of aggregated p.α-syn branches and the unstained core area observed in CLB (Figure 5B). Finally, two-color STORM imaging of LN reveals the internal organization of neurites, which contain aggregated p.α-syn cores bound to neurofilaments (Figure 5C). The first STORM images of these p.α-syn aggregates offer promising for characterizing the composition and spatial organization of Lewy pathology in the human brain.
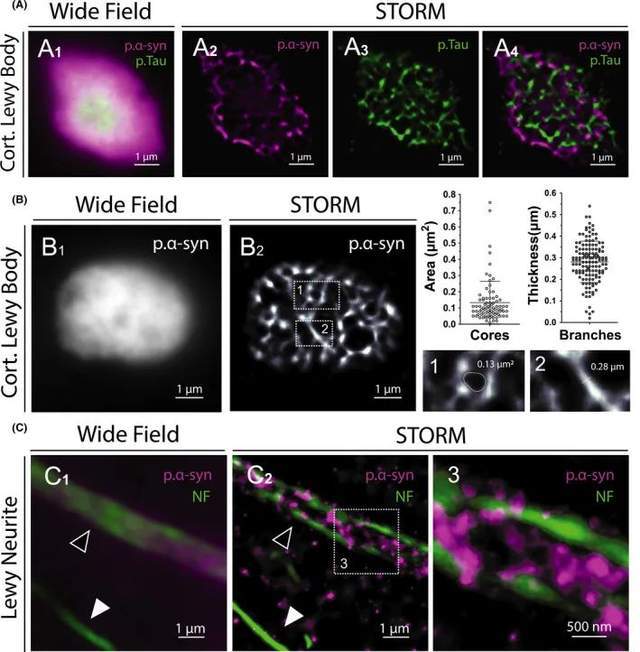 Figure 5. Colocalization and ultrastructural analysis of Lewy bodies and Lewy neurites
Figure 5. Colocalization and ultrastructural analysis of Lewy bodies and Lewy neurites
NO.2 Research summary
In this work, the researchers combined super-resolution microscopy and neuropathological techniques to analyze human brain slices. This strategy characterizes the structure of physiological structures such as axons and synapses at <50 nm resolution and images Aβ, Tau, α-synuclein, and TDP-43 pathological aggregates in samples from patients with neurodegenerative diseases in unprecedented detail.
To date, the main method for imaging nanostructures in tissues has relied on transmission electron microscopy, a time-consuming technique that requires ultrathin tissue sections (50-70 nm) with rigorous sample preparation and limits immunotargeted diversity and 3D acquisition. In contrast, STORM offers the benefits of optical fluorescence microscopy in sample preparation, wide viewing fields, multimolecular markers, and 3D acquisition, with image acquisition and reconstruction in minutes. The sample preparation workflow developed in this work for human brain tissue observation is both time-saving and easily reproducible.
Bicolor STORM allows visualization of the molecular structure of synapses, precise identification of pre- and postsynaptic protein clusters and defining their size, morphology and orientation. STORM has also been used to image pathological Aβ aggregates in mouse models of Alzheimer's disease, with results comparable to our results in humans, both in plaque morphology and Aβ fibril width, ranging from 50 to 300 nm Finally, STORM imaging of α-synuclein aggregates in mouse models of Parkinson's disease allows visualization of α-synuclein aggregation in dopaminergic neurons.
STORM imaging with fixed human tissue samples opens the door to a more complete understanding of human brain tissue and uncovering underlying mechanisms of common neurological disorders by revealing new structures and features that have not previously been visualized. The convenience of this technology should open up super-resolution imaging of human brain samples for direct expansion of the STORM app, providing promising new avenues for current neuroscience challenges.
References
1. Codron, P., F. Letournel, S. Marty, L. Renaud, A. Bodin, M. Duchesne, C. Verny, G. Lenaers, C. Duyckaerts, J. P. Julien, J. Cassereau, and A. Chevrollier. 2021. 'STochastic Optical Reconstruction Microscopy (STORM) reveals the nanoscale organization of pathological aggregates in human brain', Neuropathology and applied neurobiology, 47: 127-42.
In this study, researchers mainly use 3D-PALM super-resolution microscope to study RNAPII, 3D-PALM and 3D-STORM principle is similar, at present, in China, random optical reconstruction microscope STORM has been successfully commercialized, there are experts and teachers who need STORM imaging technology for experimental research, please fill in the questionnaire at the end of the text, you can make an appointment to get iSTORM super-resolution microscopy imaging system test shooting service~

iSTORM, the ultra-high-resolution microscopic imaging system released by Lixian Intelligence, has successfully achieved a breakthrough in the diffraction limit of optical microscopy, making it possible to engage in the research of single-molecule localization and counting of biological macromolecules, subcellular and macromolecular complex structure analysis, and biodynamics of biological macromolecules on the resolution scale of 20 nm, thus bringing major breakthroughs to life sciences, medicine and other fields.
About us
Ningbo Lixian Intelligent Technology Co., Ltd. (INVIEW) is a scientific and technological enterprise specializing in ultra-high resolution microscopy technology and product research and development, relying on the automatic control, new generation information technology of Fudan University and the biology, optics, image processing and other technologies of the Hong Kong University of Science and Technology, with optics, biology, automatic control, machinery, information technology and other interdisciplinary technical teams, the 2014 Nobel Prize in Chemistry technology industrialization, launched the ultra-high resolution microscopy imaging system iSTORM, A series of products such as the cell intelligent monitoring assistant Celeston Micro help people observe the microscopic world from an unprecedented perspective, break through the limit, and see things like never before.


