The stochastic optical reconstruction microscopy (STORM) is a super-resolution microscope with a resolution more than 10 times higher than that of traditional optical microscopes.
We know that light microscopes, with their advantages of non-contact and non-damage, have long been an important tool for biomedical research. However, due to the diffraction of light limiting the resolution of light microscopy, traditional microscopy is no longer suitable for ultrastructural imaging in life science research. This article will conduct relevant research on the random optical reconstruction microscope STORM from the aspects of principle and application, if the expression is incorrect or not in place, please correct the teacher.
01 The diffraction of light limits the resolution of the optical microscope
Before you can understand STORM, you need to know a concept. It is well known that light microscopy uses visible light to observe biological samples. Light is a transverse wave, when it passes through a circular hole, and the size of this round hole is not much different from the wavelength of light, the light will not travel in a straight line at this time, but "slip away" in all directions. In the process of light propagation, when encountering obstacles or small holes, the light will deviate from the path of linear propagation and travel around the obstacle, which is called light diffraction.
The resulting circular hole diffraction pattern is called the "Airy spot" (Figure 1). Because of this, no microscope system can concentrate light in the image plane into infinitely small points, but can only form airy spots of limited size. If the two points are very close, like the two Airy spots on the plane almost coincide, then the two points on the plane of things are indistinguishable.

Figure 1: Conceptual diagram of "Airy Spot"
Therefore, the diffraction of light makes the resolution of optical microscopes have a limit (about 200 nm), so that traditional microscopes cannot clearly observe biological structures within 200 nm, which greatly restricts the development of life science research.
02 Super-resolution microscope breaks the resolution limit
In order to see the fine structure of finer life forms, researchers must find ways to break through this imaging barrier. For this reason, a variety of super-resolution microscopes have been developed (exceeding the resolution limit of optical microscopy, so it is called super-resolution microscopy). Here, we focus on one such microscope with a comparative advantage: the stochastic optical reconstruction microscope STORM.
In Nature in 2006, Zhuang Xiaowei and other colleagues discovered a luminescent molecular cluster that can be used hundreds of times under various colors of light and can be converted in fluorescence and dark states, resulting in a microscopy technique with more than 10 times higher resolution than traditional light microscopes, and named it random optical reconstruction microscope, or STORM for short.
As mentioned earlier, two points of light close together will make us unable to tell who is who, so what if we look separately?
That is to say, when we irradiate and observe the first point, the second point does not emit light, naturally does not produce Airy spots to affect our observation of the first point, the center point position of the former Airy spot is the exact position of fluorescent molecules. Next, by some means, let the second point be illuminated. At this time, the first point is not within the illumination range of the spot, and it will not interfere with the observation of the second point. Through this "time for space" design, the constraints of the Abbe limit (microscope resolution limit) are cleverly bypassed, and the resolution of the optical microscope is greatly improved.
The STORM technique uses this idea, using organic fluorescent molecules on dyes and making a small number of fluorescent molecules in the cell glow in some way, but not all. In this way, because the distribution of luminescent points is scattered and overlapping is relatively small, each halo can be approximated as a fluorescent molecule. In one excitation, the center of one part of the halo can be determined, and in the next excitation, the center of another part of the halo can be determined, and the results of these many excitations can be superimposed to make a complete and clear image.
The STROM imaging process consists of a series of image cycles. In each cycle, only a portion of the fluorophore under the field of view is turned on so that each active fluorophore is resolved and their images are separated from other molecules without overlapping. In this way, the exact position of the group is determined, and the process is repeated many times, each time randomly opening the different subunits of the fluorophore, obtaining an image, determining the position of each subunit, and reconstructing the above image into a clear overall image. Theoretically, STORM can obtain fluorescence images with a resolution of several nanometers.
Stochastic Light Reconstructions Microscopy (STORM) is a super-resolution microscopy technique capable of imaging in two or three dimensions, in multiple colors, and even on live cells. The approach to this imaging technique, which varies greatly depending on what is being imaged, how it is imaged, and the type of image being produced, can be applied to many areas of the life sciences and provides very high-resolution images for many different needs, from neuroscience to subcellular science. Since the introduction of STORM technology, more and more researchers have recognized the advantages of this technology and widely used it in research.
(Take some recent research results as an example)
In 2013, a study in the journal Cell reported that Zhuang's team used super-resolution fluorescence imaging (STORM) to image telomere DNA in situ, directly visualize the structure of the T loop in chromatin, and systematically evaluate the role of protective proteins in the formation of the T loop.
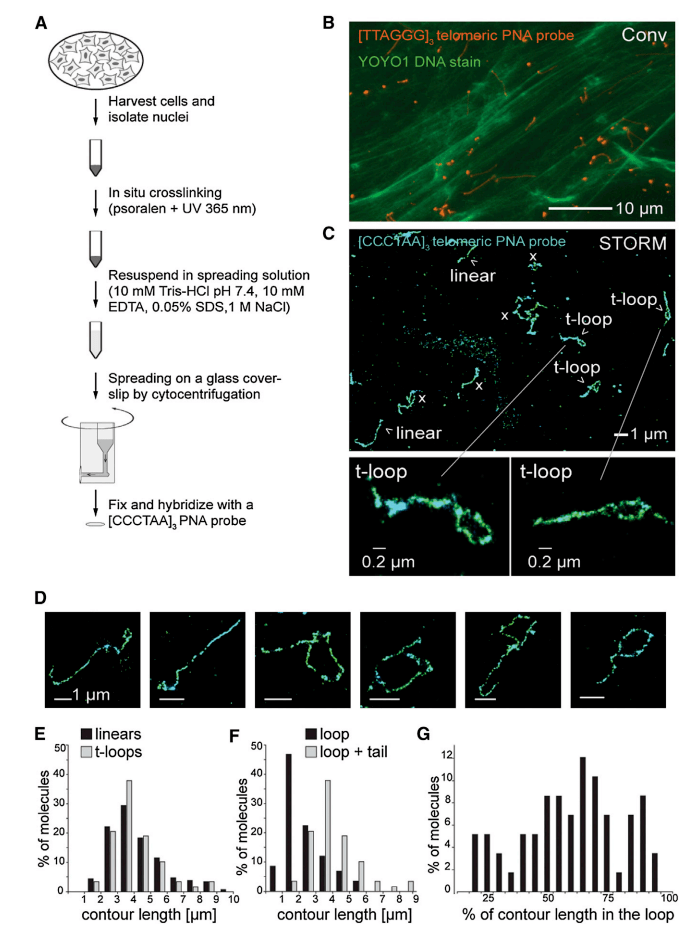
Figure 3, STORM imaging showing the T-ring after chromatin diffusion
In 2017, Liu Riyue's team developed a photobleaching method to effectively reduce the autofluorescence of cyanobacteria and plant cells and achieved a lateral resolution of ~10 nm in spherical cyanobacteria prochlorophyllum and flowering plant Arabidopsis thaliana using STORM technology.
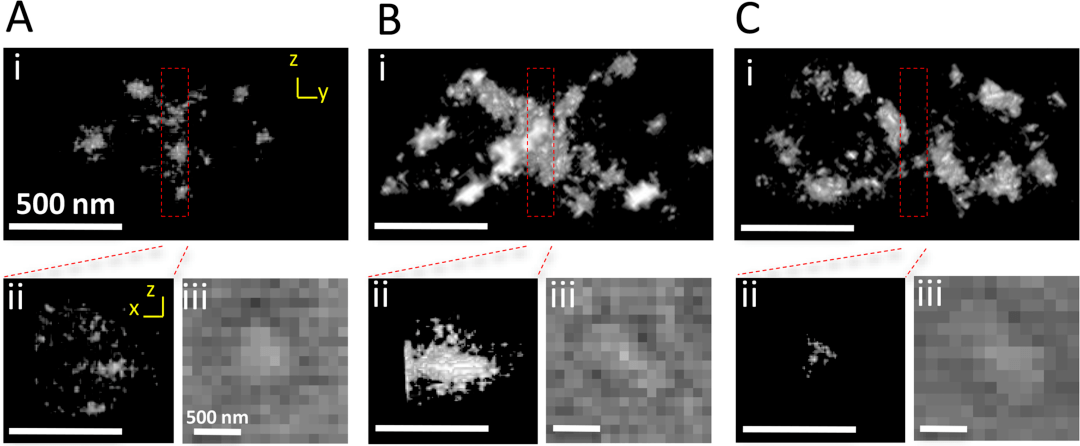
Figure 4: STORM image of the double Z ring
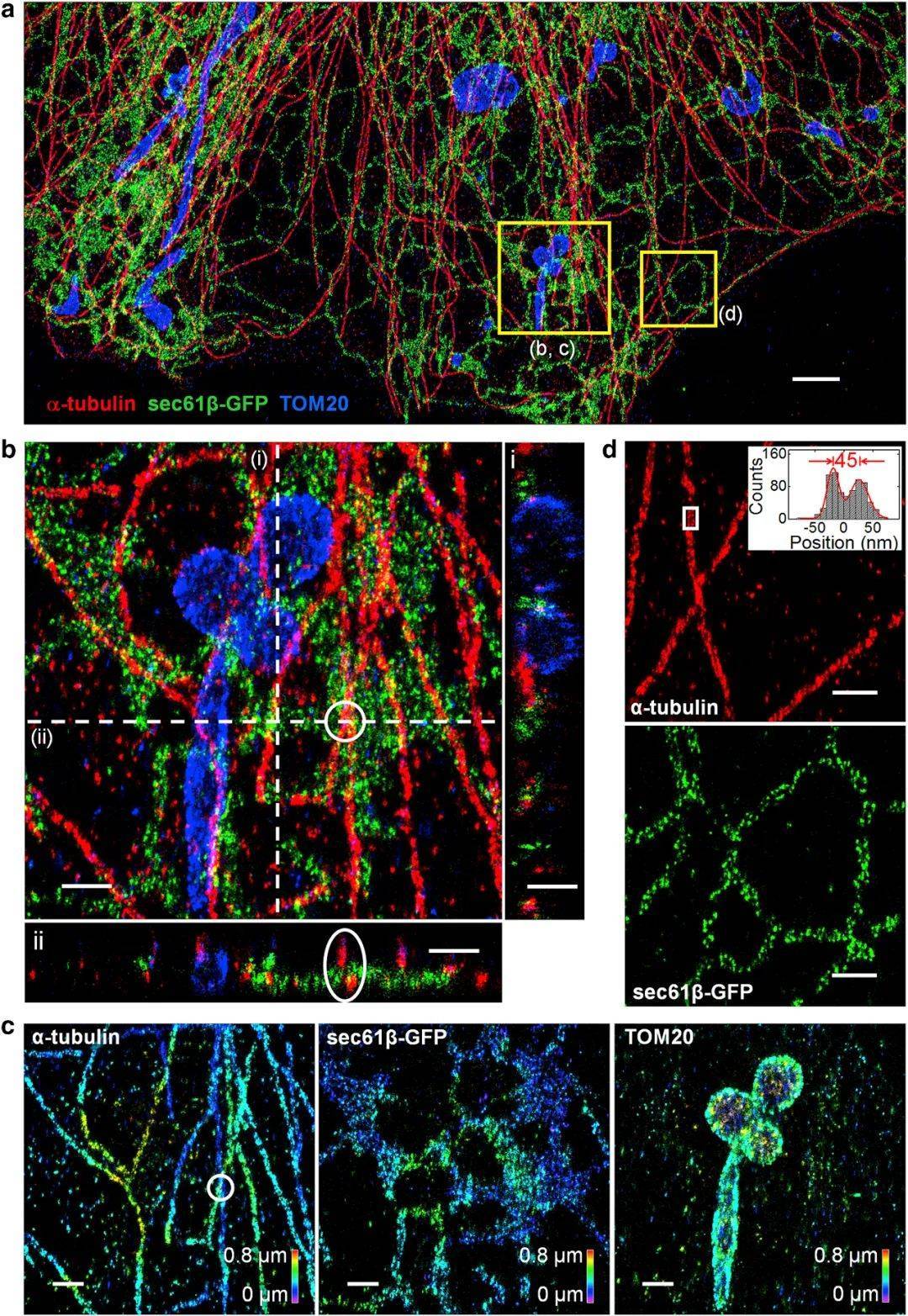
Figure 5, Three-channel, extended depth 3D prSTORM image of microtubules, mitochondria, and endoplasmic reticulum in COS-7 cells
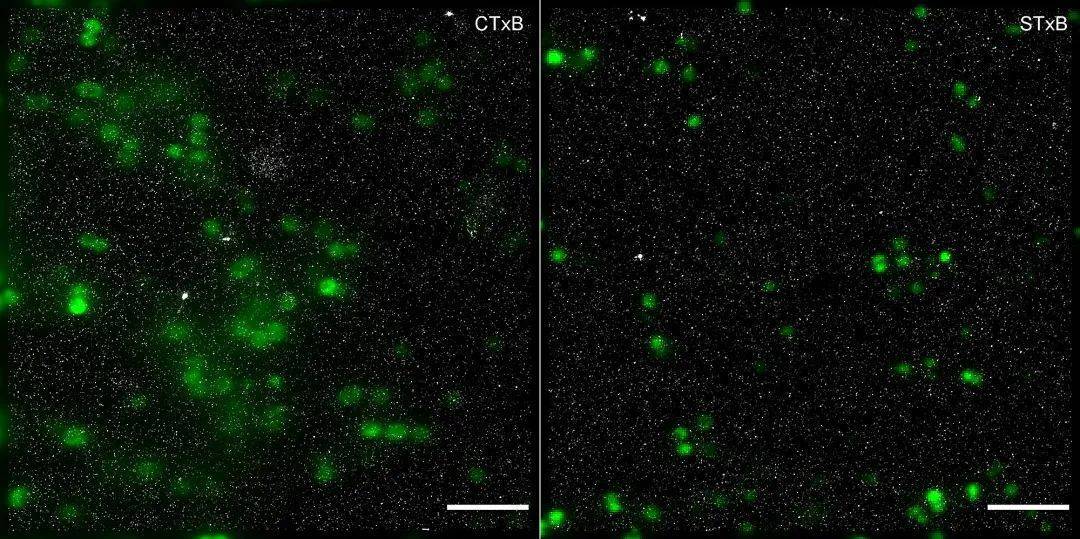
Figure 6: dSTORM images of GM1 and Gb3 of meningococci (green) expressing GFP
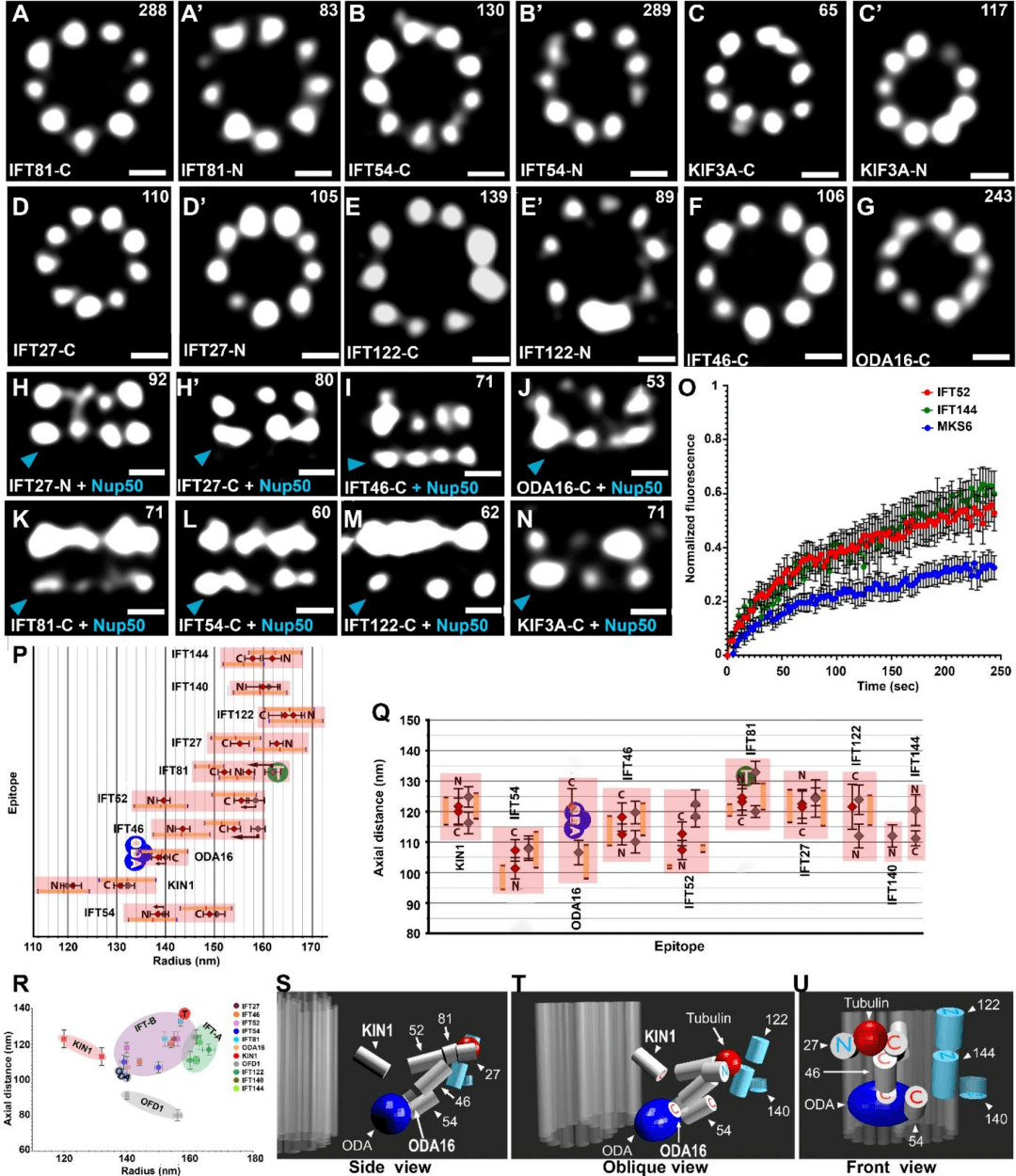
Figure 7: Localization of IFT granulin, STORM images of top and side (side) views of N- or C-terminal 3HA-labeled IFT protein, KIN1/KIF3A kinesin, and adaptor protein ODA16.
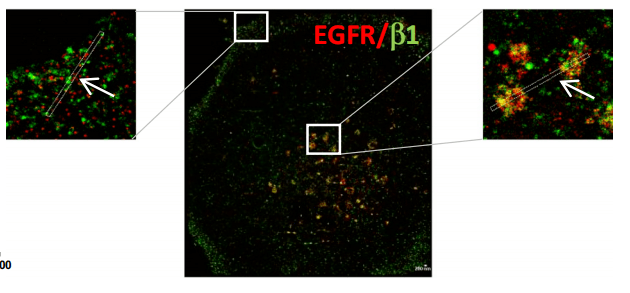
Figure 8, Two-color dSTORM image of gefitinib-treated cells showing EGFR/β1 integrin complex on peripheral and endonuclear bodies
05超高分辨显微成像系统iSTORM
The released super-resolution microscopic imaging system iSTORM has successfully achieved a breakthrough in the diffraction limit of optical microscopy, making it possible to engage in single-molecule localization and counting of biological macromolecules, subcellular and supramolecular structure analysis, and biodynamics of biological macromolecules at a resolution scale of 20 nm, thus bringing major breakthroughs to life sciences, medicine and other fields.

The ultra-high resolution microscopy imaging system iSTORM has the characteristics of 20 nm ultra-high resolution, 3-channel simultaneous imaging, 3D synchronous shooting, real-time reconstruction, and 2-hour novice mastery, and has achieved single-molecule localization and counting of live cells, and provides overall solutions for fluorescent dye selection, sample preparation, imaging services and experimental protocols, which has been highly recognized for its excellent characteristics such as nanoscale observation accuracy, high stability, wide environmental applicability, rapid imaging, and easy operation.
References:
1、Rust M J, Bates M, Zhuang X. Sub-diffraction-limit imaging by stochastic optical reconstruction microscopy (STORM)[J]. Nature methods, 2006, 3(10): 793-796.
2、Doksani Y, Wu JY, de Lange T, Zhuang X. Super-resolution fluorescence imaging of telomeres reveals TRF2-dependent T-loop formation. Cell. 2013 Oct 10;155(2):345-356. doi: 10.1016/j.cell.2013.09.048. PMID: 24120135; PMCID: PMC4062873.
3、Liu, Riyue, et al. "Three-dimensional superresolution imaging of the FtsZ ring during cell division of the cyanobacterium Prochlorococcus." MBio 8.6 (2017): e00657-17.
4、Lin, Danying, et al. "Extended-depth 3D super-resolution imaging using Probe-Refresh STORM." Biophysical journal 114.8 (2018): 1980-1987.
5、Schlegel J , Peters S , Doose S , et al. Super-Resolution Microscopy Reveals Local Accumulation of Plasma Membrane Gangliosides at Neisseria meningitidis Invasion Sites[J]. Frontiers in Cell and Developmental Biology, 2019, 7.
6、Hazime, K.S., Zhou, Z., Joachimiak, E. et al. STORM imaging reveals the spatial arrangement of transition zone components and IFT particles at the ciliary base in Tetrahymena. Sci Rep 11, 7899 (2021).
7、Blandin, Anne-Florence, et al. "Gefitinib induces EGFR and α5β1 integrin co-endocytosis in glioblastoma cells." Cellular and Molecular Life Sciences 78.6 (2021): 2949-2962.


