
01Research background
Super-resolution microscopy technology provides sub-diffraction-limited resolution that is 2 to 10 times higher than traditional confocal microscopy. This breakthrough in optical microscopy resolution has contributed to new discoveries in neuroscience and synaptic biology. Structured illumination microscopy (SIM), stimulated emission loss microscopy (STED), and random optical reconstruction microscopy (STORM)/single molecule localization microscopy (SMLM) techniques are reviewed and compared to better understand the differences and their applicability in synaptic biology analysis. The practical aspects of these microscopy techniques are also discussed, including resolution, image acquisition speed, multicolor capability, and other advantages and disadvantages. In addition, the article summarizes how super-resolution microscopy can be used to analyze neuromuscular junctions and synapses.
Neuromuscular junctions (NMJs) are large synapses that form between motor neurons and muscle fibers. Due to their large size and accessibility, these synapses have been extensively studied by researchers using histological and physiological methods. Even though the study of these synapses has been very long, the recent emergence and use of super-resolution microscopy has led to new discoveries in this type of research.
02Research Introduction (Excerpt)
First, the advantages and disadvantages of SIM, STED and STORM super-resolution microscopes
1. Resolution
Super-resolution microscopy provides sub-diffraction-limited resolution that is superior to that of confocal microscopy of approximately 200 nm. In neurobiology, confocal microscopy can image axons and nerves, dendritic morphology, mitochondrial morphology, nuclei, synapses, and NMJ. However, super-resolution microscopy can be used to image the detailed structure of spines, protein distribution patterns in Ranvier nodes, subsynaptic localization of nucleopore proteins, presynaptic and postsynaptic proteins, and synaptic vesicles.
In the case of NMJ, for example, the frontal view of NMJ is large, ranging in size from about 10 – 60 μm (human, mouse); However, some key features of this synapse are less than 200 nm diffraction limit resolution in size. For example, synaptic vesicles are 55 nm, active zones are 50 – 100 nm, and synaptic gaps are 50 – 100 nm; In addition, the junction fold peak width is 207 nm, the opening width is 55 nm (Figure 1), the laminin length is 77 nm, and the Lrp4 protein length is approximately 60 nm. Therefore, analyzing these features requires increasing the resolution of super-resolution microscopy.
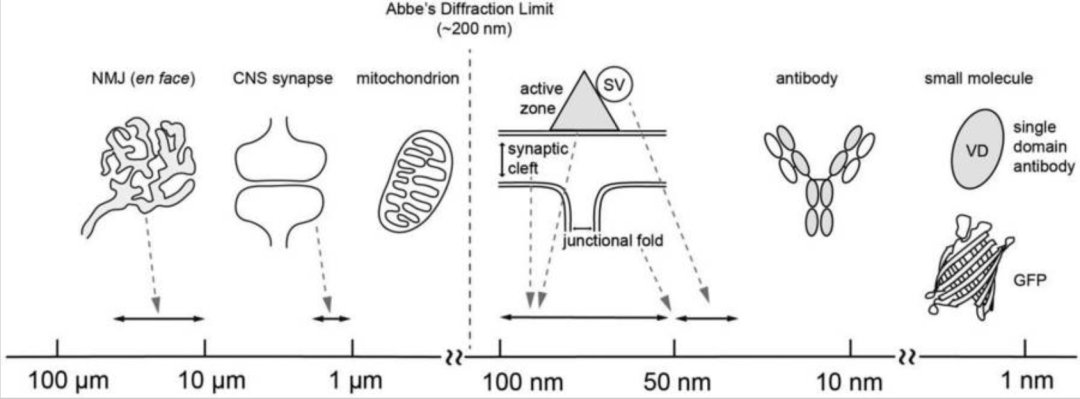
Figure 1, Abbe's diffraction limit, size of synaptic structure, and tools for super-resolution microscopy
The XY plane resolutions for these three techniques are as follows: SIM, 120 nm, STED and STORM, 20 – 30 nm (Table 1). They have significantly improved resolution compared to confocal microscopy, which has a resolution of about 200 nm in the XY plane and 500 nm along the Z axis (for green fluorescent dyes observed using NA=1.4 lenses). Because of the wide focal area, confocal microscopes have lower Z-resolution than XY resolution. The Z-axis resolutions for super-resolution microscopy techniques are as follows: SIM, 300 nm, STED, 75 – 100 nm (Easy 3D, STED 3×) and STORM, 50 nm (STORM 3D).
In addition to the difference in resolution, the three technologies also differ in the mechanism by which resolution is increased. SIM and STORM reconstruct super-resolution images based on post-processing of raw data micrographs, and STED microscopes generate super-resolution images using Fourier transforms applied to raw data micrographs. STED microscopy generates super-resolution images by scanning a laser beam over a sample, similar to confocal microscopy. STORM produces super-resolution images by collecting thousands of photomicrographs and reconstructing them using Gaussian distribution approximations and assignment points, similar to pointillism.
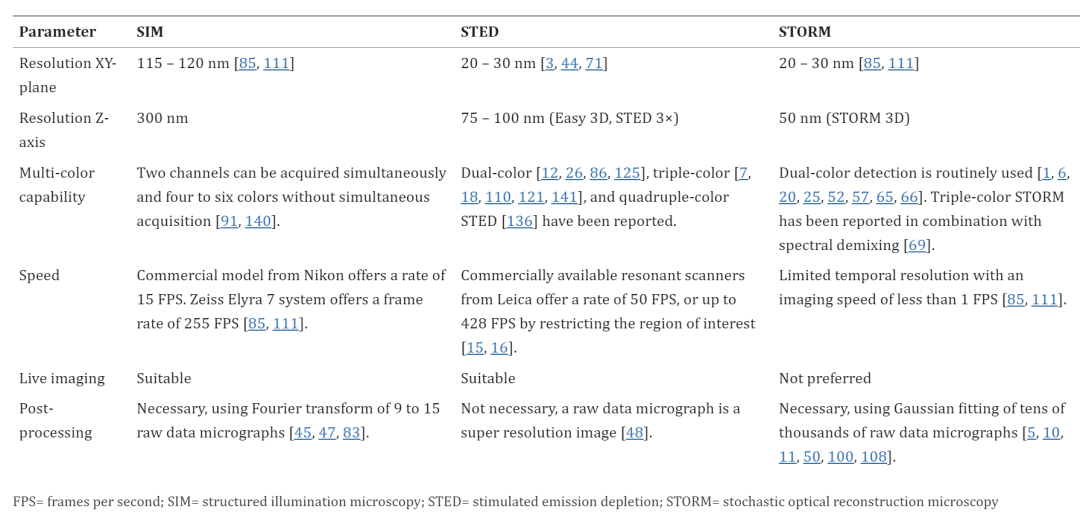
Table 1: Comparison of super-resolution microscope specifications
2. Number of colors
Similar to traditional fluorescence microscopy techniques, super-resolution microscopy can be performed using a variety of colors. Image construction for the SIM relies on post-processing of 9 to 15 acquired images with moiré stripes; Therefore, multicolor imaging can be performed if the excitation and emission wavelengths are separated between fluorophores.
In STED microscopy, an image similar to a confocal microscope is generated by scanning the sample with an excitation laser and acquiring the emission wavelength of each fluorophore. The difference from confocal microscopy is that the emission wavelength of the fluorophore needs to be matched to the wavelength of the STED laser to deplete the excited fluorophore to the ground state.
STORM requires fluorophores that switch between the non-emitted state and the ground state under high-intensity light, as well as a dedicated buffer environment for these switched fluorophores. The fluorophore randomly relaxes to the ground state and is then excited to emit fluorescence. Fluorophores with these properties are called photoswitchable (photoscintillation) dyes (for STORM) and photoswitchable fluorescent proteins (for PALM). The dSTORM or activator-free STORM method allows the use of commercially available photoswitchable dyes conjugated to secondary antibodies without the need for activator-reporter-pair dyes, which facilitates multicolor analysis. Tricolor STORM analysis resolves fluorophores with overlapping emission spectra by combining STORM with spectral separation (spectral unmixing) methods.
3. Imaging speed and real-time imaging
Commercial SIM microscopes have a similar temporal resolution to STED microscopes and are superior to STORM because the number of raw photomicrographs required for SIM reconstruction of super-resolution images is only 9 to 15, while STORM requires tens of thousands. In addition, SIM requires a lower imaging light intensity compared to STED and STORM. SIM live imaging is currently used to analyze a variety of nervous system structures, including growth cones of cultured neurons, growth cone cytoskeletons and vesicles, dendritic spines of cultured hippocampal neurons, and dendritic spines in the brain, live zebrafish larvae, or live mice.
STED microscopy is similar to confocal microscopy, both are scanning microscopes. Spot scanning microscopy allows imaging of smaller areas or lines to improve temporal resolution rather than scanning the entire field of view. STED microscopes enable fast real-time imaging at video rates; For example, 38.5 FPS is used to analyze vesicle fusion and endocytosis, and 28 FPS is used to analyze synaptic proteins in neurons.
THE STORM (PALM, SMLM) IMAGING SPEED IS ABOUT 0.1 FPS BECAUSE TENS OF THOUSANDS OF FRAMES (PHOTOMICROGRAPHS) ARE REQUIRED TO RECONSTRUCT SUPER-RESOLUTION IMAGES, WHICH IS LOWER THAN THE TEMPORAL RESOLUTION OF SIM AND STED. Currently, several algorithms have been developed to improve the temporal resolution of STORM, including a multi-emitter fitting algorithm that identifies molecular positions that may have overlapping point diffusion functions. STORM has been combined with TIRF microscopy to have shallow imaging depths below 1 micron, and sections from a few microns to 30 microns thick can be imaged with STORM.
At present, various super-resolution microscopy techniques can basically be commercialized, but each technology has advantages and disadvantages, and it needs to be considered comprehensively before choosing a method for the project. For example, if a project requires the highest resolution, STORM and STED can provide the best resolution available for commercially available super-resolution microscopes with lateral resolution of 20 to 30 nm. If a project requires protein quantity information, STORM has been shown to provide quantitative information.
Second, the applicability of super-resolution microscope types to synaptic analysis
1. SIM for NMJ analysis
Three-dimensional structured illumination microscopy 3D-SIM has been used to analyze the role of large adaptor Ankyrin2-L in stabilizing Drosophila larvae NMJ by regulating presynaptic microtubules and cell adhesion molecules. 3D-SIM reveals a repeating lattice structure with a periodicity of about 200 nm consisting of Ankyrin2-L and β-spectrin in axons, but poorly organized in presynaptic boutons.
The distribution patterns of synaptic proteins, including the presynaptic active zone protein Piccolo and postsynaptic protein rapsyn, voltage-gated sodium channels, and integrin α7, were analyzed relative to the distribution patterns of postsynaptic junction folding by using 3D-SIM in mouse NMJ. Consistent with previous reports, these proteins were observed at the expected location of NMJ.
2. STED microscope for NMJ analysis
The first application of super-resolution microscopy in NMJ analysis was the imaging of the active zone of Drosophila NMJ using STED microscopy. The active region is the synaptic vesicle release site on the presynaptic membrane. Active regions are shown in electron microscopy images as T-bars in Drosophila NMJ or small aggregates in vertebrate NMJ. Confocal microscopy previously showed that the protein was distributed in dots at motor nerve endings, without any distinct structure within each lacrimal punctum. The subdiffraction-limited resolution of the STED microscope revealed for the first time the structure within each punctum and elucidated the circular pattern of Bruchpilot protein in each punctum.
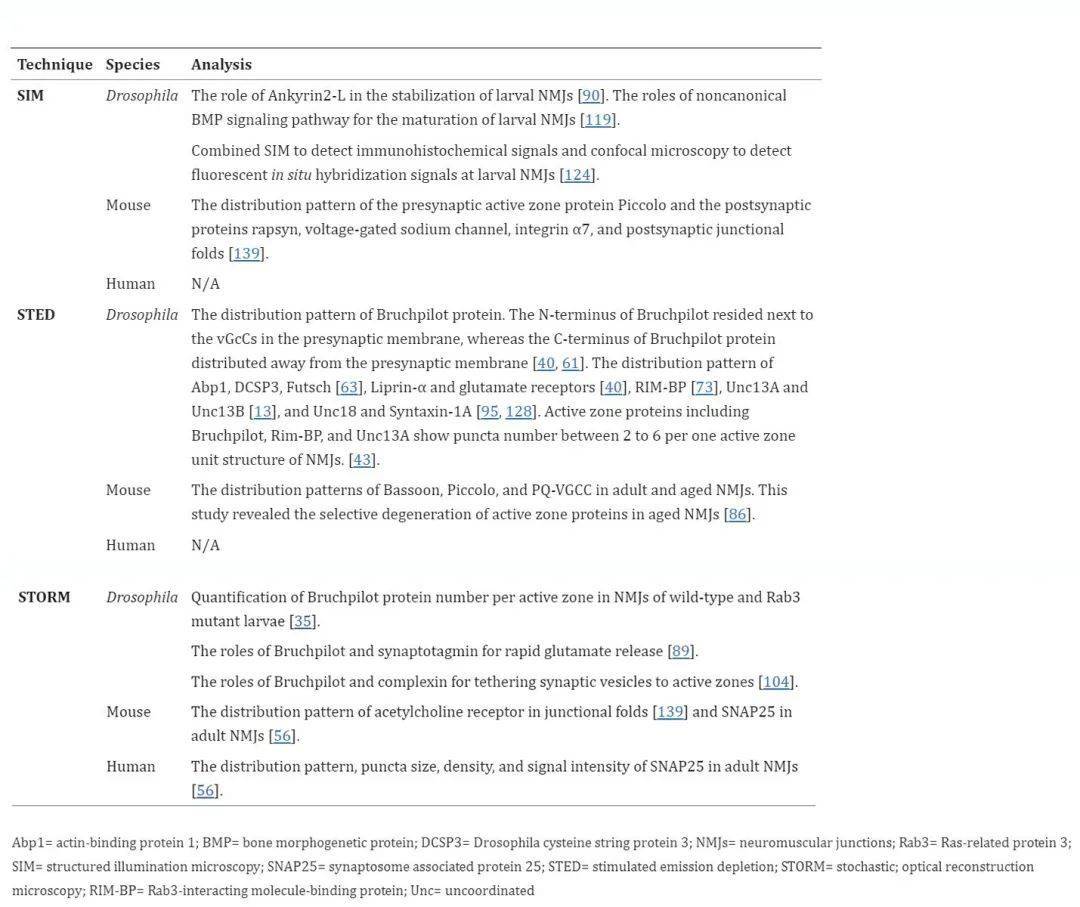
Table 2, Super-resolution microscopy techniques for NMJ analysis
3. STORM is used for NMJ analysis
Similar to STED microscopy, STORM was originally used to analyze Drosophila larvae NMJ. Quantitatively analyze the active zone of Drosophila larvae NMJ using direct stochastic optical reconstruction microscopy (dSTORM). As a single-molecule localization microscopy method, dSTORM is used to quantify endogenous proteins. The number of active region-specific Bruchpilot proteins is estimated at 137 proteins per active region, and three-quarters of these proteins are organized into approximately 15 different locations with 7 Bruchpilot proteins at each location. This quantitative dSTORM technique successfully showed an approximately 60% increase in the number of Bruchpilot protein per active region in the Rab3 mutant larvae NMJ. In addition, STORM was used to analyze the role of Bruchpilot and synaptotagmin in the release of rapid glutamate and the role of Bruchpilot and complex proteins in binding synaptic vesicles to the active zone of fruit fly larvae NMJ.
In mouse NMJs, based on STORM images, the authors present a novel pattern of acetylcholine receptor distribution in which receptor concentrations are higher at the shoulder of the junctional fold but lower at the ridge. This pattern of distribution differs slightly from the classical understanding, in which acetylcholine receptors are thought to be distributed at the top and shoulder of the junctional fold ridge, rather than at the bottom of the fold.
The authors used STORM to compare human and mouse NMJs to analyze the distribution pattern of synaptosome-associated protein 25 (SNAP25). In mouse NMJs, SNAP25 is distributed in a punctate manner with a density (15 per μm2) similar to that of the active zone proteins Bassoon and Piccolo (10 per μm2). Interestingly, human NMJs show greater SNAP25 values in punctum size, punctal density in each NMJ region, and signal intensity in immunohistochemistry compared to mouse NMJ. Based on these findings, the authors propose a difference between human and mouse NMJs.
03研究结论
Super-resolution microscopy studies of Drosophila NMJ have come a long way and have revealed impressive nanostructures and molecular properties of active regions previously known as T-bars in electron micrographs. The combination of good molecular markers (e.g., Bruchpilot of active regions) and abundant genetic mutational resources provides abundant opportunities to study the molecular mechanisms of NMJ physiological modifications in Drosophila using super-resolution microscopy. Super-resolution microscopy research on mammalian NMJs is still in its infancy. To our knowledge, these super-resolution methods have not been used for live imaging of vertebrate NMJ. However, vertebrate NMJ has been studied in frogs and mice using electron microscopy tomography, revealing ultrastructural details of presynaptic activity zones. Super-resolution microscopy offers the opportunity to reveal the molecular identities of these modeled structures in order to better understand vertebrate synapses, and the development of super-resolution microscopy has improved the understanding of the mechanisms underpinning the molecules.
04超高分辨率显微成像系统iSTORM
The stochastic optical reconstruction microscope (STORM) technology mentioned above has been successfully commercialized. The super-resolution microscopy imaging system iSTORM has successfully achieved a breakthrough in the diffraction limit of optical microscopy, making it possible to engage in single-molecule localization and counting of biological macromolecules, subcellular and supramolecular structure analysis, and biodynamics of biological macromolecules on a resolution scale of 20 nm, thus bringing major breakthroughs to life sciences, medicine and other fields.

Figure 2: iSTORM, a super-resolution microscopic imaging system.
Ultra-high resolution microscopy imaging system iSTORM has the characteristics of 20 nm ultra-high resolution, 3-channel simultaneous imaging, 3D simultaneous shooting, real-time reconstruction, and 2-hour novice mastery, etc., has realized the localization and counting of single molecules in living cells, and provides fluorescent dye selection, sample preparation, imaging services and experimental solutions as an overall solution. It has been highly recognized by more than 50 scientific research groups and more than 100 researchers.
References:
Badawi Y, Nishimune H. Super-resolution microscopy for analyzing neuromuscular junctions and synapses. Neurosci Lett. 2020 Jan 10;715:134644. doi: 10.1016/j.neulet.2019.134644. Epub 2019 Nov 22. PMID: 31765730; PMCID: PMC6937598.


