
01Research Introduction (Excerpt)
Diagnosing kidney disease is complex and challenging, often requiring the use of light microscopy (LM), immunohistology, and electron microscopy (EM). EM is useful in the histopathology of ~50% of natural kidney biopsies and important for ~20% of diagnoses, making EM the standard technique for natural kidney biopsy in many countries. EM is commonly used to diagnose kidney disease associated with structural abnormalities of the basement membrane, fibrillary disorders, and rare genetic disorders. It is also frequently used to record morphological changes in podocytes and to record the shape, substructure, and location of immune complex or complement fragment deposits relative to the glomerular basement membrane (GBM).
However, EM instruments are currently unavailable to most people in the world, and the number of specialists and facilities where they can be used is decreasing. It is important for developing and developed countries to find a cheaper alternative to EM that enhances diagnostic capabilities, surpasses LM and IF, reduces the number of instruments required, and simplifies and accelerates diagnostic workflows.
In recent years, optical microscopy has broken through the diffraction limit through super-resolution microscopy (SRM) techniques, such as structured illumination microscopy (SIM), random switching single molecule localization microscopy (SMLM), photoactivated localization microscopy, and random optical reconstruction microscopy (STORM).
This is shown by the technique of direct stochastic optical reconstruction microscopy (dSTORM). In a previous approach the authors call "easySTORM", the authors have demonstrated that dSTORM can use multimode diode lasers and multimode fiber at a relatively low cost (<£20,000) in a large field of view (>). 120 × 120 μm) stably provides super-resolution images. The authors show that this approach can be applied to clinical histological sections to provide super-resolution IF imaging using clinically approved antibodies. The authors describe it as a "histoSTORM" approach.
In particular, the authors explore the possibility of replacing EM with histoSTORM in the diagnosis of kidney disease, perhaps providing a clinical tool that is less expensive and can be widely used. This follows earlier work using SIM and STORM to study nephropod substructure and protein organization in GBM. While previous work demonstrated the potential of super-resolved IF, it was achieved with expensive commercial SRM instruments, and studies utilizing STORM were conducted with mouse tissue and non-clinically approved antibodies.
The authors' goal is to develop a low-cost method that allows clinicians in most countries to use easySTORM to image clinically relevant proteins, and to develop practical protocols using existing clinically validated antibodies to process existing biopsy samples, such as cryosections or paraffin wax (FFPE) sections. The authors note that STORM has previously been applied to pathology studies, such as to study epigenetic regulation and cancer progression.
02 Research results
In the initial small study, the authors applied histoSTORM with standard clinically approved antibodies to FFPE and frozen tissue sections. The authors believe that the exemplary results presented below show clinically meaningful ultrastructures that may be useful for diagnosis.
Membranous glomerulonephritis (Figure 1) is characterized by a subepithelial immune complex deposit containing IgG in the basement membrane and thickening of the structure. The two-color wide-field IF image in Figure 1D shows a capillary ring with GBM in green with an anti-human laminin probe (green, Alexa Fluor 555) and IgG deposits on the epithelial side of the filter barrier in red (IgG, iFluor 647). The dSTORM image displayed at 25 nm per pixel shows well-defined subepithelial deposits consistent with the image observed by EM (Figure 1F, I). Although there is no clear structural definition in the wide-field IF image (Figure 1D, G), the details of immunodeposits in the subepithelial region and the gradient of immunodeposited content are easily observed in dSTORM (Figure 1E, H). The green region of the dSTORM image represents the GBM region without immune deposits, the yellow region represents the overlap of laminin and immune deposits, and the red region represents the immune deposit cluster.
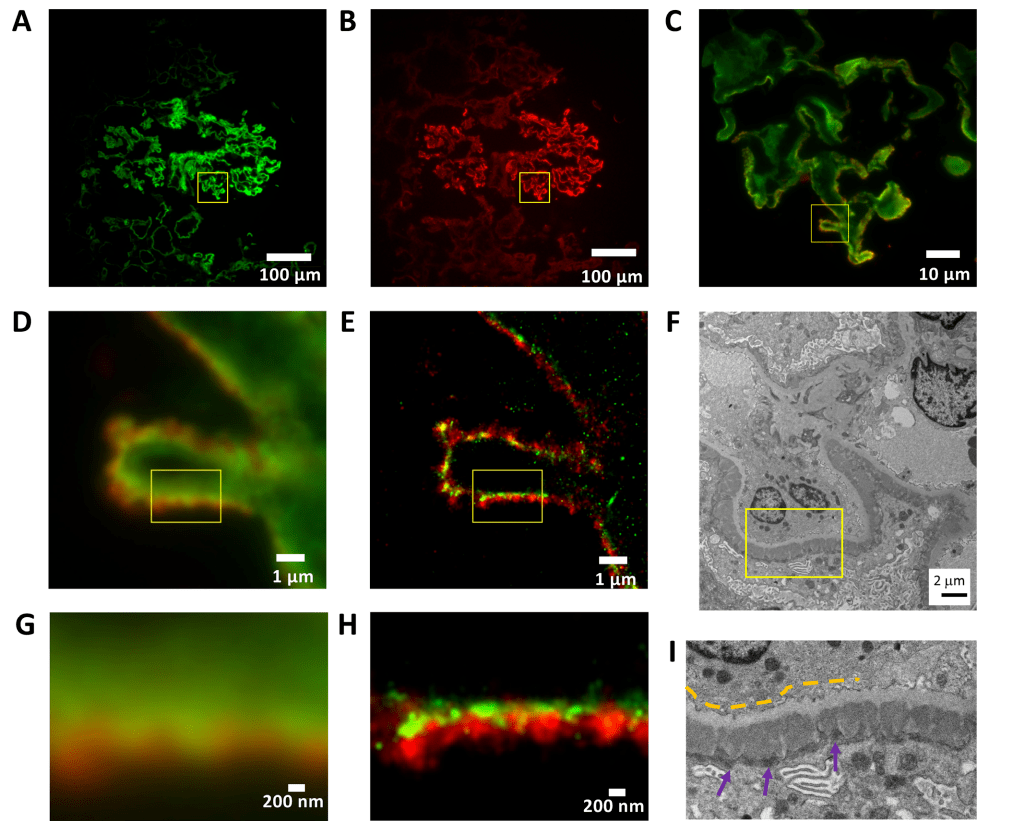
Figure 1: Membranous glomerulonephritis. Basement membrane (laminin, green - Alexa Fluor 555) and IgG deposits (red - iFluor 647).
(A–C) membranous glomerulonephritis cryosection× 100x magnified wide-field IF image display.
(A) Laminin channel.
(B) IgG channel.
(C) Extended two-channel image of the area indicated by the yellow square in (A) and (B).
(D) Wide field of view IF, for the area represented by the yellow square in (C).
(E) The corresponding STORM image with a pixel size of 25 nm.
(F) Obtain electron microscope photographs of similar structures from the same biopsy at ×5,500x magnification.
(G) 3.2 × 2.4 μm 2-region wide-field IF image indicated in (D) and (E).
(H) The corresponding STORM image.
(I) Extended electron microscope image in the area shown in (F).
The yellow dotted line indicates a light gray GBM. Dark gray electron-dense deposits (purple arrows) on the underside of the epithelium represent immune complexes containing IgG.
Lupus nephritis is characterized by the deposition of polyclonal IgG glomeruli in different areas of the glomerulus. Figure 2 shows the deposition of IgG (red, iFluor 647) in glomerular capillaries in stage IV lupus nephritis and basement membrane staining (laminin, green, Alexa Fluor 555). At this stage, mesangial (Figure 2G), subendothelial (Figure 2H), and subepithelial (Figure 2I) IgG deposits are easily observed with dSTORM and summarize the distribution of high electron density IgG deposits recorded with EM (Figure 2C).
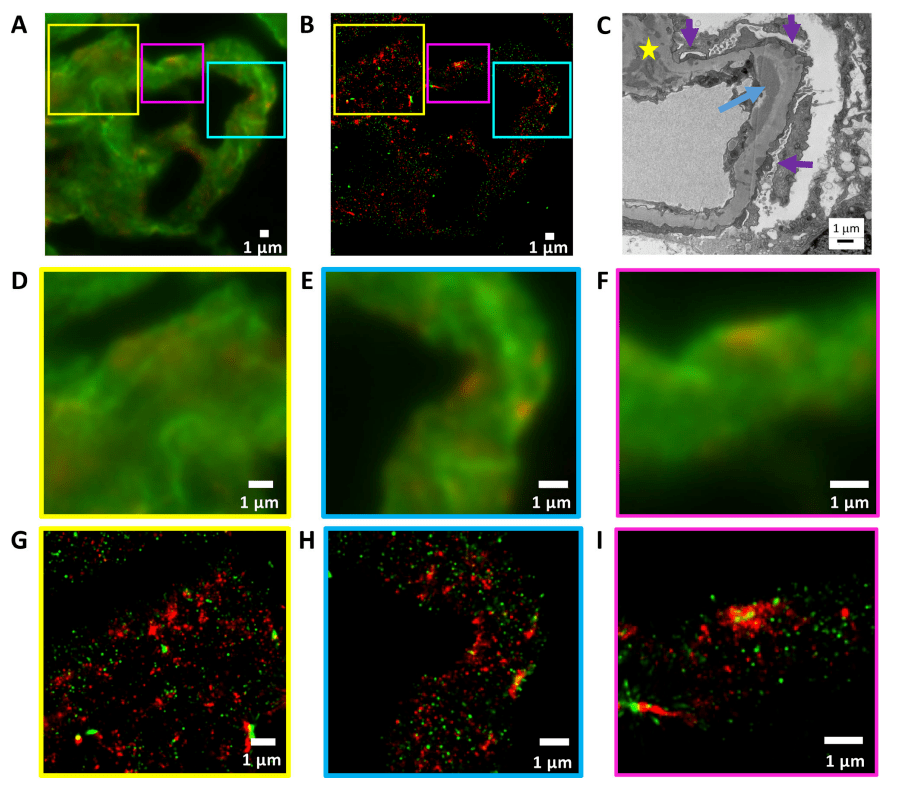
Figure 2, lupus nephritis type IV. Basement membrane (laminin, green - Alexa Fluor 555) and IgG deposits (red - iFluor 647).
(A) Cryosection× wide field IF image at 100 magnification, showing lupus nephritis type IV with (D,G) mesangial deposits, (E,H) subepithelial deposits, and (F,I) subepithelial deposits in the selected area.
(B) corresponds to (A), a STORM image displayed at 25 nm pixel size.
(C) Obtain electron micrographs of similar structures from the same sample at ×8000x magnification, occasionally showing electron-dense deposits containing IgG on the inferior epithelium of GBM (purple arrow), on the inferior endothelium of BBM (blue arrow) and mesangium (yellow star).
(G–I) A STORM image corresponding to a wide-field IF image (D–F).
(D) and (G) show the area indicated by the yellow squares in (A) and (B).
(E) and (H) show the area indicated by the cyan squares in (A) and (B).
(F) and (I) show the area indicated by the purple squares in (A) and (B).
easySTORM provides a large field of view (120 μm × 120 μm) with a resolution below the diffraction limit, allowing to assess GBM thickness in glomerular capillaries and record other aspects of the glomerulus. Figures 3A, C show wide-field epifluorescence images of FFPE sections from micro-change disease biopsies, where laminin in GBM is labeled with iFluor 647. Figures 3B and D show the corresponding STORM images and confirm the ability of the STORM images to measure GBM thickness below the diffraction limit. Figures 3F and G show the wide field of view through the GBM and the line segments of the STORM image. Figure 3E shows an electron microscope photograph with a GBM width of 281 nm.
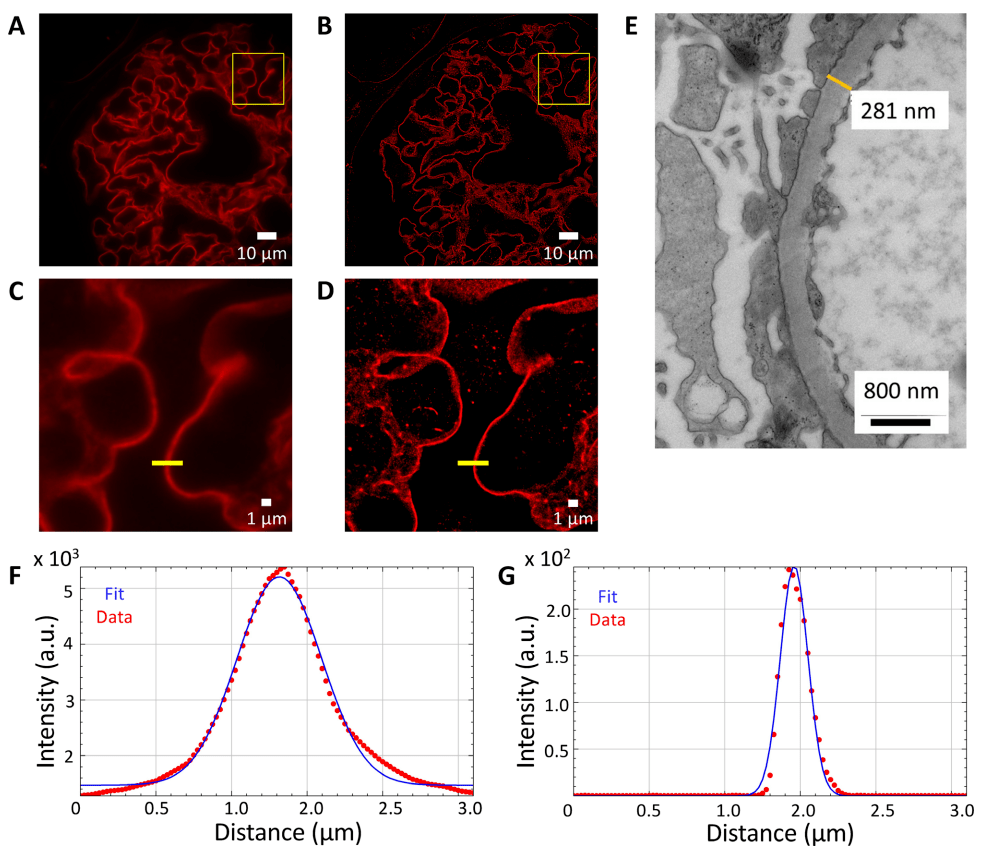
Figure 3: Minor change disease: GBM thickness measurement (lamin-iFluor 647).
(A) A wide-field IF image with a wide field of view magnification of ×100x in the FFPE section.
Rendered storm image of the area shown in (B)(A).
Illustration of the wide field of view of the area shown in the yellow box in (C)(A).
STORM illustration of the area shown in the yellow box in (D)(B) with a pixel size of 25 nm.
(E) Electron microscopic photographs of GBM taken at × 15500x magnification from different parts of the same biopsy with a GBM thickness of 281 nm at the designated location.
(F) GBM thickness (half-height full width) measured from a widefield IF image (C) at the yellow line (657 nm).
(G) Measured thickness (half-height full width) of the storm image (D) at the yellow line (212 nm).
03超高分辨显微成像系统iSTORM
At present, in China, the random optical reconstruction microscope STORM has been successfully commercialized, and the super-resolution microscopy imaging system iSTORM has successfully achieved a breakthrough in the diffraction limit of optical microscopy, making it possible to engage in the research of single-molecule localization and counting of biological macromolecules, subcellular and supramolecular structure analysis, and biodynamics of biological macromolecules on the resolution scale of 20 nm, thus bringing major breakthroughs to life sciences, medicine and other fields.

The ultra-high resolution microscopy imaging system iSTORM has the characteristics of 20nm ultra-high resolution, 3-channel simultaneous imaging, 3D simultaneous shooting, real-time reconstruction, 2-hour novice mastery, etc., and provides fluorescent dye selection, sample preparation, imaging services and experimental solutions as an overall solution, which has been highly recognized by more than 50 scientific research groups and more than 100 researchers for its excellent characteristics such as nanoscale observation accuracy, high stability, wide environmental applicability, rapid imaging, and easy operation.
References
Garcia E, Lightley J, Kumar S, Kalita R, Gőrlitz F, Alexandrov Y, Cook T, Dunsby C, Neil MA, Roufosse CA, French PM. Application of direct stochastic optical reconstruction microscopy (dSTORM) to the histological analysis of human glomerular disease. J Pathol Clin Res. 2021 Sep;7(5):438-445. doi: 10.1002/cjp2.217. Epub 2021 May 21. PMID: 34018698; PMCID: PMC8363924.


